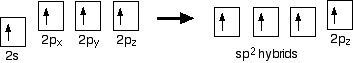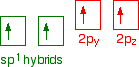ELECTRONIC STRUCTURE AND ATOMIC ORBITALS A simple view In any introductory chemistry course you will have come across the electronic structures of hydrogen and carbon drawn as: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note: There are many places where you could still make use of this model of the atom at A' level. It is, however, a simplification and can be misleading. It gives the impression that the electrons are circling the nucleus in orbits like planets around the sun. As you will see in a moment, it is impossible to know exactly how they are actually moving. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The circles show energy levels - representing increasing distances from the nucleus. You could straighten the circles out and draw the electronic structure as a simple energy diagram. Atomic orbitals Orbits and orbitals sound similar, but they have quite different meanings. It is essential that you understand the difference between them. The impossibility of drawing orbits for electrons To plot a path for something you need to know exactly where the object is and be able to work out exactly where it's going to be an instant later. You can't do this for electrons. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note: In order to plot a plane's course, it is no use knowing its exact location in mid-Atlantic if you don't know its direction or speed. Equally it's no use knowing that it is travelling at 500 mph due west if you have no idea whether it is near Iceland or the Azores at that particular moment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Heisenberg Uncertainty Principle (not required at A'level) says - loosely - that you can't know with certainty both where an electron is and where it's going next. That makes it impossible to plot an orbit for an electron around a nucleus. Is this a big problem? No. If something is impossible, you have to accept it and find a way around it. Hydrogen's electron - the 1s orbital | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note: In this diagram (and the orbital diagrams that follow), the nucleus is shown very much larger than it really is. This is just for clarity. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
You keep on doing this over and over again, and gradually build up a sort of 3D map of the places that the electron is likely to be found. In the hydrogen case, the electron can be found anywhere within a spherical space surrounding the nucleus. The diagram shows across-section through this spherical space. 95% of the time (or any other percentage you choose), the electron will be found within a fairly easily defined region of space quite close to the nucleus. Such a region of space is called an orbital.You can think of an orbital as being the region of space in which the electron lives. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note: If you wanted to be absolutely 100% sure of where the electron is, you would have to draw an orbital the size of the Universe! | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
What is the electron doing in the orbital? We don't know, we can't know, and so we just ignore the problem! All you can say is that if an electron is in a particular orbital it will have a particular definable energy. Each orbital has a name. The orbital occupied by the hydrogen electron is called a 1s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. The "s" tells you about the shape of the orbital. s orbitals are spherically symmetric around the nucleus - in each case, like a hollow ball made of rather chunky material with the nucleus at its centre.
If you look carefully, you will notice that there is another region of slightly higher electron density (where the dots are thicker) nearer the nucleus. ("Electron density" is another way of talking about how likely you are to find an electron at a particular place.) 2s (and 3s, 4s, etc) electrons spend some of their time closer to the nucleus than you might expect. The effect of this is to slightly reduce the energy of electrons in s orbitals. The nearer the nucleus the electrons get, the lower their energy. 3s, 4s (etc) orbitals get progressively further from the nucleus. p orbitals Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. A p orbital is rather like 2 identical balloons tied together at the nucleus. The diagram on the right is a cross-section through that 3-dimensional region of space. Once again, the orbital shows where there is a 95% chance of finding a particular electron. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Beyond A'level: If you imagine a horizontal plane through the nucleus, with one lobe of the orbital above the plane and the other beneath it, there is a zero probability of finding the electron on that plane. So how does the electron get from one lobe to the other if it can never pass through the plane of the nucleus? For A'level chemistry you just have to accept that it does! If you want to find out more, read about the wave nature of electrons. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Unlike an s orbital, a p orbital points in a particular direction - the one drawn points up and down the page. At any one energy level it is possible to have three absolutely equivalent p orbitals pointing mutually at right angles to each other. These are arbitrarily given the symbols px, py and pz. This is simply for convenience - what you might think of as the x, y or z direction changes constantly as the atom tumbles in space.
All levels except for the first level have p orbitals. At the higher levels the lobes get more elongated, with the most likely place to find the electron more distant from the nucleus. Fitting electrons into orbitals Because for the moment we are only interested in the electronic structures of hydrogen and carbon, we don't need to concern ourselves with what happens beyond the second energy level.
Each orbital can hold either 1 or 2 electrons, but no more. "Electrons-in-boxes" Orbitals can be represented as boxes with the electrons in them shown as arrows. Often an up-arrow and a down-arrow are used to show that the electrons are in some way different. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Beyond A'level: The need to have all electrons in an atom different comes out of quantum theory. If they live in different orbitals, that's fine - but if they are both in the same orbital there has to be some subtle distinction between them. Quantum theory allocates them a property known as "spin" - which is what the arrows are intended to suggest. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
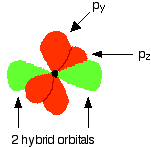
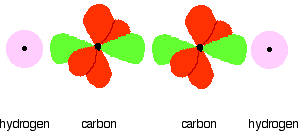
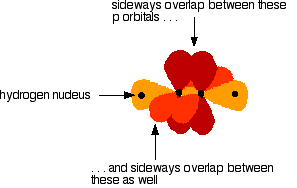
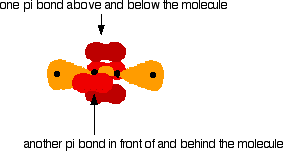
You mustn't confuse the two numbers in this notation: The order of filling orbitals Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. Where there is a choice between orbitals of equal energy, they fill the orbitals singly as far as possible. The diagram (not to scale) summarises the energies of the various orbitals in the first and second levels. Notice that the 2s orbital has a slightly lower energy than the 2p orbitals. That means that the 2s orbital will fill with electrons before the 2p orbitals. All the 2p orbitals have exactly the same energy. The electronic structure of hydrogen Hydrogen only has one electron and that will go into the orbital with the lowest energy - the 1s orbital. Hydrogen has an electronic structure of 1s1. We have already described this orbital earlier. The electronic structure of carbon Carbon has six electrons. Two of them will be found in the 1s orbital close to the nucleus. The next two will go into the 2s orbital. The remaining ones will be in two separate 2p orbitals. This is because the p orbitals all have the same energy and the electrons prefer to be on their own if that's the case. BONDING IN METHANE AND ETHANE Methane, CH4 The simple view of the bonding in methane You will be familiar with drawing methane using dots and crosses diagrams, but it is worth looking at its structure a bit more closely. There is a serious mis-match between this structure and the modern electronic structure of carbon, 1s22s22px12py1. The modern structure shows that there are only 2 unpaired electrons for hydrogens to share with, instead of the 4 which the simple view requires. You can see this more readily using the electrons-in-boxes notation. Only the 2-level electrons are shown. The 1s2electrons are too deep inside the atom to be involved in bonding. The only electrons directly available for sharing are the 2p electrons. Why then isn't methane CH2? Promotion of an electron When bonds are formed, energy is released and the system becomes more stable. If carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even more stable. There is only a small energy gap between the 2s and 2p orbitals, and so it pays the carbon to provide a small amount of energy to promote an electron from the 2s to the empty 2p to give 4 unpaired electrons. The extra energy released when the bonds form more than compensates for the initial input. The carbon atom is now said to be in an excited state. Now that we've got 4 unpaired electrons ready for bonding, another problem arises. In methane all the carbon-hydrogen bonds are identical, but our electrons are in two different kinds of orbitals. You aren't going to get four identical bonds unless you start from four identical orbitals. Hybridisation The electrons rearrange themselves again in a process called hybridisation. This reorganises the electrons into four identical hybrid orbitals called sp3hybrids (because they are made from one s orbital and three p orbitals). You should read "sp3" as "s p three" - not as "s p cubed". sp3 hybrid orbitals look a bit like half a p orbital, and they arrange themselves in space so that they are as far apart as possible. You can picture the nucleus as being at the centre of a tetrahedron (a triangularly based pyramid) with the orbitals pointing to the corners. For clarity, the nucleus is drawn far larger than it really is. What happens when the bonds are formed? Remember that hydrogen's electron is in a 1s orbital - a spherically symmetric region of space surrounding the nucleus where there is some fixed chance (say 95%) of finding the electron. When a covalent bond is formed, the atomic orbitals (the orbitals in the individual atoms) merge to produce a new molecular orbital which contains the electron pair which creates the bond. Four molecular orbitals are formed, looking rather like the original sp3 hybrids, but with a hydrogen nucleus embedded in each lobe. Each orbital holds the 2 electrons that we've previously drawn as a dot and a cross. The principles involved - promotion of electrons if necessary, then hybridisation, followed by the formation of molecular orbitals - can be applied to any covalently-bound molecule. The shape of methane When sp3 orbitals are formed, they arrange themselves so that they are as far apart as possible. That is a tetrahedral arrangement, with an angle of 109.5°. Nothing changes in terms of the shape when the hydrogen atoms combine with the carbon, and so the methane molecule is also tetrahedral with 109.5° bond angles. Ethane, C2H6 The formation of molecular orbitals in ethane Ethane isn't particularly important in its own right, but is included because it is a simple example of how a carbon-carbon single bond is formed. Each carbon atom in the ethane promotes an electron and then forms sp3 hybrids exactly as we've described in methane. So just before bonding, the atoms look like this: The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. The two carbon atoms bond by merging their remaining sp3 hybrid orbitals end-to-end to make a new molecular orbital. The bond formed by this end-to-end overlap is called a sigma bond. The bonds between the carbons and hydrogens are also sigma bonds. In any sigma bond, the most likely place to find the pair of electrons is on a line between the two nuclei. The shape of ethane around each carbon atom The shape is again determined by the way the sp3 orbitals are arranged around each carbon atom. That is a tetrahedral arrangement, with an angle of 109.5°. When the ethane molecule is put together, the arrangement around each carbon atom is again tetrahedral with approximately 109.5° bond angles. Why only "approximately"? This time, each carbon atoms doesn't have four identical things attached. There will be a small amount of distortion because of the attachment of 3 hydrogens and 1 carbon, rather than 4 hydrogens. Free rotation about the carbon-carbon single bond The two ends of this molecule can spin quite freely about the sigma bond so that there are, in a sense, an infinite number of possibilities for the shape of an ethane molecule. Some possible shapes are: In each case, the left hand CH3 group has been kept in a constant position so that you can see the effect of spinning the right hand one. Other alkanes All other alkanes will be bonded in the same way: The carbon atoms will each promote an electron and then hybridise to give sp3 hybrid orbitals. Ethene, C2H4 The simple view of the bonding in ethene At a simple level, you will have drawn ethene showing two bonds between the carbon atoms. Each line in this diagram represents one pair of shared electrons. Ethene is actually much more interesting than this. An orbital view of the bonding in ethene Ethene is built from hydrogen atoms (1s1) and carbon atoms (1s22s22px12py1). The carbon atom doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s2 pair into the empty 2pz orbital. This is exactly the same as happens whenever carbon forms bonds - whatever else it ends up joined to. So the first thing that happens is . . . Promotion of an electron There is only a small energy gap between the 2s and 2p orbitals, and an electron is promoted from the 2s to the empty 2p to give 4 unpaired electrons. The extra energy released when these electrons are used for bonding more than compensates for the initial input. The carbon atom is now said to be in an excited state. Hybridisation In the case of ethene, there is a difference from, say, methane or ethane, because each carbon is only joining to three other atoms rather than four. When the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise three of the orbitals rather than all four. They use the 2s electron and two of the 2p electrons, but leave the other 2p electron unchanged. The new orbitals formed are calledsp2 hybrids, because they are made by an s orbital and two p orbitals reorganising themselves. sp2 orbitals look rather like sp3 orbitals that you have already come across in the bonding in methane, except that they are shorter and fatter. The three sp2 hybrid orbitals arrange themselves as far apart as possible - which is at 120° to each other in a plane. The remaining p orbital is at right angles to them. The two carbon atoms and four hydrogen atoms would look like this before they joined together: The various atomic orbitals which are pointing towards each other now merge to give molecular orbitals, each containing a bonding pair of electrons. These aresigma bonds - just like those formed by end-to-end overlap of atomic orbitals in, say, ethane. The p orbitals on each carbon aren't pointing towards each other, and so we'll leave those for a moment. In the diagram, the black dots represent the nuclei of the atoms. Notice that the p orbitals are so close that they are overlapping sideways. This sideways overlap also creates a molecular orbital, but of a different kind. In this one the electrons aren't held on the line between the two nuclei, but above and below the plane of the molecule. A bond formed in this way is called a pi bond. For clarity, the sigma bonds are shown using lines - each line representing one pair of shared electrons. The various sorts of line show the directions the bonds point in. An ordinary line represents a bond in the plane of the screen (or the paper if you've printed it), a broken line is a bond going back away from you, and a wedge shows a bond coming out towards you. Be clear about what a pi bond is. It is a region of space in which you can find the two electrons which make up the bond. Those two electrons can live anywhere within that space. It would be quite misleading to think of one living in the top and the other in the bottom. Even if your syllabus doesn't expect you to know how a pi bond is formed, it will expect you to know that it exists. The pi bond dominates the chemistry of ethene. It is very vulnerable to attack - a very negative region of space above and below the plane of the molecule. It is also somewhat distant from the control of the nuclei and so is a weaker bond than the sigma bond joining the two carbons. If you are working to a UK-based syllabus for 16 - 18 year olds, and haven't got a copy of your syllabus, find out how to download one All double bonds (whatever atoms they might be joining) will consist of a sigma bond and a pi bond. The shape of ethene The shape of ethene is controlled by the arrangement of the sp2orbitals. Notice two things about them: They all lie in the same plane, with the other p orbital at right angles to it. When the bonds are made, all of the sigma bonds in the molecule must also lie in the same plane. Any twist in the molecule would mean that the p orbitals wouldn't be parallel and touching any more, and you would be breaking the pi bond. There is no free rotation about a carbon-carbon double bond. Ethene is a planar molecule. The sp2 orbitals are at 120° to each other. When the molecule is constructed, the bond angles will also be 120°. (That's approximate! There will be a slight distortion because you are joining 2 hydrogens and a carbon atom to each carbon, rather than 3 identical groups.) Ethyne, C2H2 The simple view of the bonding in ethyne Ethyne has a triple bond between the two carbon atoms. In the diagram each line represents one pair of shared electrons. If you have read the ethene page, you will expect that ethyne is going to be more complicated than this simple structure suggests. An orbital view of the bonding in ethyne Ethyne is built from hydrogen atoms (1s1) and carbon atoms (1s22s22px12py1). The carbon atom doesn't have enough unpaired electrons to form four bonds (1 to the hydrogen and three to the other carbon), so it needs to promote one of the 2s2 pair into the empty 2pz orbital. This is exactly the same as happens whenever carbon forms bonds - whatever else it ends up joined to. Each carbon is only joining to two other atoms rather than four (as in methane or ethane) or three (as in ethene) and so when the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise two of the orbitals. They use the 2s electron and one of the 2p electrons, but leave the other 2p electrons unchanged. The new hybrid orbitals formed are called sp1 hybrids (sometimes just sp hybrids), because they are made by an s orbital and a single p orbital reorganising themselves. What these look like in the atom (using the same colour coding) is: Notice that the two green lobes are two different hybrid orbitals - arranged as far apart from each other as possible. Don't confuse them with the shape of a p orbital. The two carbon atoms and two hydrogen atoms would look like this before they joined together: The various atomic orbitals which are pointing towards each other now merge to give molecular orbitals, each containing a bonding pair of electrons. These are sigma bonds - just like those formed by end-to-end overlap of atomic orbitals in, say, ethane. The sigma bonds are shown as orange in the next diagram. The various p orbitals (now shown in slightly different reds to avoid confusion) are now close enough together that they overlap sideways. Sideways overlap between the two sets of p orbitals produces two pi bonds - each similar to the pi bond found in, say, ethene. These pi bonds are at 90° to each other - one above and below the molecule, and the other in front of and behind the molecule. Notice the different shades of red for the two different pi bonds. BONDING IN BENZENE The Kekulé structure for benzene, C6H6 What is the Kekulé structure? Kekulé was the first to suggest a sensible structure for benzene. The carbons are arranged in a hexagon, and he suggested alternating double and single bonds between them. Each carbon atom has a hydrogen attached to it. This diagram is often simplified by leaving out all the carbon and hydrogen atoms! In diagrams of this sort, there is a carbon atom at each corner. You have to count the bonds leaving each carbon to work out how many hydrogens there are attached to it. In this case, each carbon has three bonds leaving it. Because carbon atoms form four bonds, that means you are a bond missing - and that must be attached to a hydrogen atom. Problems with the Kekulé structure Although the Kekulé structure was a good attempt in its time, there are serious problems with it . . . Problems with the chemistry Because of the three double bonds, you might expect benzene to have reactions like ethene - only more so! Ethene undergoes addition reactions in which one of the two bonds joining the carbon atoms breaks, and the electrons are used to bond with additional atoms. Benzene rarely does this. Instead, it usually undergoes substitution reactions in which one of the hydrogen atoms is replaced by something new. Problems with the shape Benzene is a planar molecule (all the atoms lie in one plane), and that would also be true of the Kekulé structure. The problem is that C-C single and double bonds are different lengths. That would mean that the hexagon would be irregular if it had the Kekulé structure, with alternating shorter and longer sides. In real benzene all the bonds are exactly the same - intermediate in length between C-C and C=C at 0.139 nm. Real benzene is a perfectly regular hexagon. Problems with the stability of benzene Real benzene is a lot more stable than the Kekulé structure would give it credit for. Every time you do a thermochemistry calculation based on the Kekulé structure, you get an answer which is wrong by about 150 kJ mol-1. This is most easily shown using enthalpy changes of hydrogenation. Hydrogenation is the addition of hydrogen to something. If, for example, you hydrogenate ethene you get ethane: In order to do a fair comparison with benzene (a ring structure) we're going to compare it with cyclohexene. Cyclohexene, C6H10, is a ring of six carbon atoms containing just one C=C. When hydrogen is added to this, cyclohexane, C6H12, is formed. The "CH" groups become CH2 and the double bond is replaced by a single one. The structures of cyclohexene and cyclohexane are usually simplified in the same way that the Kekulé structure for benzene is simplified - by leaving out all the carbons and hydrogens. In the cyclohexane case, for example, there is a carbon atom at each corner, and enough hydrogens to make the total bonds on each carbon atom up to four. In this case, then, each corner represents CH2. The hydrogenation equation could be written: The enthalpy change during this reaction is -120 kJ mol-1. In other words, when 1 mole of cyclohexene reacts, 120 kJ of heat energy is evolved. Where does this heat energy come from? When the reaction happens, bonds are broken (C=C and H-H) and this costs energy. Other bonds have to be made, and this releases energy. Because the bonds made are stronger than those broken, more energy is released than was used to break the original bonds and so there is a net evolution of heat energy. If the ring had two double bonds in it initially (cyclohexa-1,3-diene), exactly twice as many bonds would have to be broken and exactly twice as many made. In other words, you would expect the enthalpy change of hydrogenation of cyclohexa-1,3-diene to be exactly twice that of cyclohexene - that is, -240 kJ mol-1. In fact, the enthalpy change is -232 kJ mol-1 - which isn't far off what we are predicting. Applying the same argument to the Kekulé structure for benzene (what might be called cyclohexa-1,3,5-triene), you would expect an enthalpy change of -360 kJ mol-1, because there are exactly three times as many bonds being broken and made as in the cyclohexene case. In fact what you get is -208 kJ mol-1 - not even within distance of the predicted value! This is very much easier to see on an enthalpy diagram. Notice that in each case heat energy is released, and in each case the product is the same (cyclohexane). That means that all the reactions "fall down" to the same end point. Heavy lines, solid arrows and bold numbers represent real changes. Predicted changes are shown by dotted lines and italics. The most important point to notice is that real benzene is much lower down the diagram than the Kekulé form predicts. The lower down a substance is, the more energetically stable it is. This means that real benzene is about 150 kJ mol-1 more stable than the Kekulé structure gives it credit for. This increase in stability of benzene is known as the delocalisation energy orresonance energy of benzene. The first term (delocalisation energy) is the more commonly used. Why is benzene so much more stable than the Kekulé structure suggests? To explain that needs a separate article! Follow the first link below. An orbital model for the benzene structure Building the orbital model Benzene is built from hydrogen atoms (1s1) and carbon atoms (1s22s22px12py1). Each carbon atom has to join to three other atoms (one hydrogen and two carbons) and doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s2 pair into the empty 2pz orbital. So the first thing that happens is . . . Promotion of an electron There is only a small energy gap between the 2s and 2p orbitals, and an electron is promoted from the 2s to the empty 2p to give 4 unpaired electrons. The extra energy released when these electrons are used for bonding more than compensates for the initial input. The carbon atom is now said to be in an excited state. Hybridisation Because each carbon is only joining to three other atoms, when the carbon atoms hybridise their outer orbitals before forming bonds, they only need to hybridise three of the orbitals rather than all four. They use the 2s electron and two of the 2p electrons, but leave the other 2p electron unchanged. The new orbitals formed are calledsp2 hybrids, because they are made by an s orbital and two p orbitals reorganising themselves. The three sp2 hybrid orbitals arrange themselves as far apart as possible - which is at 120° to each other in a plane. The remaining p orbital is at right angles to them. Each carbon atom now looks like the diagram on the right. This is all exactly the same as happens in ethene. The difference in benzene is that each carbon atom is joined to two other similar carbon atoms instead of just one. Each carbon atom uses the sp2 hybrids to form sigma bonds with two other carbons and one hydrogen atom. The next diagram shows the sigma bonds formed, but for the moment leaves the p orbitals alone. Only a part of the ring is shown because the diagram gets extremely cluttered if you try to draw any more. Notice that the p electron on each carbon atom is overlapping with those on both sides of it. This extensive sideways overlap produces a system of pi bonds which are spread out over the whole carbon ring. Because the electrons are no longer held between just two carbon atoms, but are spread over the whole ring, the electrons are said to be delocalised. The six delocalised electrons go into three molecular orbitals - two in each. In common with the great majority of descriptions of the bonding in benzene, we are only going to show one of these delocalised molecular orbitals for simplicity. In the diagram, the sigma bonds have been shown as simple lines to make the diagram less confusing. The two rings above and below the plane of the molecule represent onemolecular orbital. The two delocalised electrons can be found anywhere within those rings. The other four delocalised electrons live in two similar (but not identical) molecular orbitals. Relating the orbital model to the properties of benzene The shape of benzene Benzene is a planar regular hexagon, with bond angles of 120°. This is easily explained. It is a regular hexagon because all the bonds are identical. The delocalisation of the electrons means that there aren't alternating double and single bonds. It is planar because that is the only way that the p orbitals can overlap sideways to give the delocalised pi system. The energetic stability of benzene This is accounted for by the delocalisation. As a general principle, the more you can spread electrons around - in other words, the more they are delocalised - the more stable the molecule becomes. The extra stability of benzene is often referred to as "delocalisation energy". The reluctance of benzene to undergo addition reactions With the delocalised electrons in place, benzene is about 150 kJ mol-1 more stable than it would otherwise be. If you added other atoms to a benzene ring you would have to use some of the delocalised electrons to join the new atoms to the ring. That would disrupt the delocalisation and the system would become less stable. Since about 150 kJ per mole of benzene would have to be supplied to break up the delocalisation, this isn't going to be an easy thing to do. The symbol for benzene Although you will still come across the Kekulé structure for benzene, for most purposes we use the structure on the right. The hexagon shows the ring of six carbon atoms, each of which has one hydrogen attached. (You have to know that - counting bonds to find out how many hydrogens to add doesn't work in this particular case.) The circle represents the delocalised electrons. It is essential that you include the circle. If you miss it out, you are drawing cyclohexane and not benzene. The carbonyl group The simple view of the bonding in carbon - oxygen double bonds Where the carbon-oxygen double bond, C=O, occurs in organic compounds it is called acarbonyl group. The simplest compound containing this group is methanal. We are going to look at the bonding in methanal, but it would equally apply to any other compound containing C=O. The interesting thing is the nature of the carbon-oxygen double bond - not what it's attached to. Naming: methanal: meth counts 1 carbon atom, an means no C=C, al says that it is an aldehyde and so contains CHO. An orbital view of the bonding in carbon - oxygen double bonds The carbon atom Just as in ethene or benzene, the carbon atom is joined to three other atoms. The carbon's electrons rearrange themselves, and promotion and hybridisation give sp2 hybrid orbitals. Promotion gives: Hybridisation of the 2s orbital and two of the 2p orbitals means that the carbon atom now looks like the diagram on the right. Three sp2 hybrid orbitals are formed and these arrange themselves as far apart in space as they can - at 120° to each other. The remaining p orbital is at right angles to them. This is exactly the same as in ethene or in benzene. The oxygen atom Oxygen's electronic structure is 1s22s22px22py12pz1. The 1s electrons are too deep inside the atom to be concerned with the bonding and so we'll ignore them from now on. Hybridisation occurs in the oxygen as well. It is easier to see this using "electrons-in-boxes". This time two of the sp2 hybrid orbitals contain lone pairs of electrons. The carbon atom and oxygen atom then bond in much the same way as the two carbons do in ethene. In the next diagram, we are assuming that the carbon will also bond to two hydrogens to make methanal - but it could equally well bond to anything else. End-to-end overlap between the atomic orbitals that are pointing towards each other produce sigma bonds. Notice that the p orbitals are overlapping sideways. This sideways overlap produces a pi bond. So just like C=C, C=O is made up of a sigma bond and a pi bond. Does that mean that the bonding is exactly the same as in ethene? No! The distribution of electrons in the pi bond is heavily distorted towards the oxygen end of the bond, because oxygen is much more electronegative than carbon. This distortion in the pi bond causes major differences in the reactions of compounds containing carbon-oxygen double bonds like methanal compared with compounds containing carbon-carbon double bonds like ethene. This page deals with electronegativity in an organic chemistry context. If you want a wider view of electronegativity, there is a link at the bottom of the page. What is electronegativity? Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is given a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7. What happens if two atoms of equal electronegativity bond together? The most obvious example of this is the bond between two carbon atoms. Both atoms will attract the bonding pair to exactly the same extent. That means that on average the electron pair will be found half way between the two nuclei, and you could draw a picture of the bond like this: It is important to realise that this is an average picture. The electrons are actually in a sigma orbital, and are moving constantly within that orbital. The carbon-fluorine bond Fluorine is much more electronegative than carbon. The actual values on the Pauling scale are That means that fluorine attracts the bonding pair much more strongly than carbon does. The bond - on average - will look like this: Why is fluorine more electronegative than carbon? A simple dots-and-crosses diagram of a C-F bond is perfectly adequate to explain it. The bonding pair is in the second energy level of both carbon and fluorine, so in the absence of any other effect, the distance of the pair from both nuclei would be the same. The electron pair is shielded from the full force of both nuclei by the 1s electrons - again there is nothing to pull it closer to one atom than the other. BUT, the fluorine nucleus has 9 protons whereas the carbon nucleus has only 6. Allowing for the shielding effect of the 1s electrons, the bonding pair feels a net pull of about 4+ from the carbon, but about 7+ from the fluorine. It is this extra nuclear charge which pulls the bonding pair (on average) closer to the fluorine than the carbon. Incidentally, thinking about electrons looking towards the nucleus may be helpful in picturing what is going on, but avoid using terms like this in exams. The carbon-chlorine bond The electronegativities are: The bonding pair of electrons will be dragged towards the chlorine but not as much as in the fluorine case. Chlorine isn't as electronegative as fluorine. Why isn't chlorine as electronegative as fluorine? Chlorine is a bigger atom than fluorine. fluorine: 1s22s22px22py22pz1 chlorine: 1s22s22px22py22pz23s23px23py23pz1 In the chlorine case, the bonding pair will be shielded by all the 1-level and 2-level electrons. The 17 protons on the nucleus will be shielded by a total of 10 electrons, giving a net pull from the chlorine of about 7+. That is the same as the pull from the fluorine, but with chlorine the bonding pair starts off further away from the nucleus because it is in the 3-level. Since it is further away, it feels the pull from the nucleus less strongly. Bond polarity and inductive effects Polar bonds Think about the carbon-fluorine bond again. Because the bonding pair is pulled towards the fluorine end of the bond, that end is left rather more negative than it would otherwise be. The carbon end is left rather short of electrons and so becomes slightly positive. The symbols We describe a bond having one end slightly positive and the other end slightly negative as being polar. Inductive effects An atom like fluorine which can pull the bonding pair away from the atom it is attached to is said to have a negative inductive effect. Most atoms that you will come across have a negative inductive effect when they are attached to a carbon atom, because they are mostly more electronegative than carbon. You will come across some groups of atoms which have a slight positive inductive effect - they "push" electrons towards the carbon they are attached to, making it slightly negative. Inductive effects are sometimes given symbols: -I (a negative inductive effect) and +I (a positive inductive effect). Some important examples of polar bonds Hydrogen bromide (and other hydrogen halides) Bromine (and the other halogens) are all more electronegative than hydrogen, and so all the hydrogen halides have polar bonds with the hydrogen end slightly positive and the halogen end slightly negative. Halide: a compound of one of these - e.g. hydrogen chloride, hydrogen bromide, etc. The polarity of these molecules is important in their reactions with alkenes. The carbon-bromine bond in halogenoalkanes Bromine is more electronegative than carbon and so the bond is polarised in the way that we have already described with C-F and C-Cl. The polarity of the carbon-halogen bonds is important in the reactions of the halogenoalkanes. The carbon-oxygen double bond An orbital model of the C=O bond in methanal, HCHO, looks like this: The very electronegative oxygen atom pulls both bonding pairs towards itself - in the sigma bond and the pi bond. That leaves the oxygen fairly negative and the carbon fairly positive. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
About Me
Hi Everybody
Are you feeling bored with the chemical reactions that you have seen,especially the Organic Chemistry Reactions. Here are some simple illustrations of some reaction mechanisms, and What actually Chemistry is !!!
Disclaimer
All the information given in this site has been taken from other sites. Thanks to the publishers who had done this Great job. My viewers are allowed only to view this site's content. You are restricted from copying or storing any of these articles. If its done, the author of the site will bear no responsibility for that. Thank you
-
Welcome Message
Glad to see you
Followers
Blogger templates
Aug 28, 2010 | By Ajesh
Bonding in organic compounds
2010 - Have Fun With Chemistry is proudly powered by Blogger
Blogger Template created by Anshul
Design By Templatelite.com
Blogger Template created by Anshul
Design By Templatelite.com