Cope Rearrangement
(Anionic) Oxy-Cope Rearrangement
The Cope Rearrangement is the thermal isomerization of a 1,5-diene leading to a regioisomeric 1,5-diene. The main product is the thermodynamically more stable regioisomer. The Oxy-Cope has a hydroxyl substituent on an sp3-hybridized carbon of the starting isomer.
The driving force for the neutral or anionic Oxy-Cope Rearrangement is that the product is an enol or enolate (resp.), which can tautomerize to the corresponding carbonyl compound. This product will not equilibrate back to the other regioisomer.

The Oxy-Cope Rearrangement proceeds at a much faster rate when the starting alcohol is deprotonated, e.g. with KH. The reaction is then up to 1017 times faster, and may be conducted at room temperature. Aqueous work up then gives the carbonyl compound.

Mechanism of the Cope Rearrangement


Two transition states are possible, and the outcome of the reaction can be predicted on the basis of the most favorable overlap of the orbitals of the double bond, as influenced by stereoelectronic factors:


Curtius Rearrangement

The Curtius Rearrangement is the thermal decomposition of carboxylic azides to produce an isocyanate. These intermediates may be isolated, or their corresponding reaction or hydrolysis products may be obtained.
The reaction sequence - including subsequent reaction with water which leads to amines - is named the Curtius Reaction. This reaction is similar to the Schmidt Reaction with acids, differing in that the acyl azide in the present case is prepared from the acyl halide and an azide salt.

Mechanism of the Curtius Rearrangement
Preparation of azides:
Decomposition:

Reaction with water to the unstable carbamic acid derivative which will undergo spontaneous decarboxylation:


Isocyanates are versatile starting materials:

Isocyanates are also of high interest as monomers for polymerization work and in the derivatisation of biomacromolecules.
Dakin Reaction

The Dakin Reaction allows the preparation of phenols from aryl aldehydes or aryl ketones via oxidation with hydrogen peroxide in the presence of base. The aryl formate or alkanoate formed as an intermediate is subsequently saponified to yield the substituted phenol product.
Ortho or para +M substituents (NH2, OH) favor this reaction.
Mechanism of the Dakin Reaction
See Baeyer-Villiger Oxidation for the oxidation step.Darzens Reaction
Darzens Condensation
The Darzens Reaction is the condensation of a carbonyl compound with an α-halo ester in the presence of a base to form an α,β-epoxy ester.
Mechanism of the Darzens Reaction
After deprotonation, the α-halo ester adds to the carbonyl compound to give syn and anti diastereomers:In the subsequent step, an intramolecular SN2 reaction forms the epoxide:
Typically, the cis:trans ratio of the epoxide formation lies between 1:1 and 1:2.
In the past, Darzens methodology was primarily used for the synthesis of aldehydes and ketones, as a homologation reaction without any consideration of stereocontrol in the epoxide formation. For this sequence, saponification of the α,β-epoxy ester followed by decarboxylation gives the substituted carbonyl compound:
Darzens methodology for the construction of epoxides can also be used for α-halo carbonyl compounds, or similar compounds that can undergo deprotonation and bear electron-withdrawing groups. In addition, the reaction can be carried out with diazoacetate, where N2 is the leaving group, or with a sulphur ylide with SR2 as the leaving group.
Diazotisation

The nitrosation of primary aromatic amines with nitrous acid (generated in situ from sodium nitrite and a strong acid, such as hydrochloric acid, sulfuric acid, or HBF4) leads to diazonium salts, which can be isolated if the counterion is non-nucleophilic.
Diazonium salts are important intermediates for the preparation of halides (Sandmeyer Reaction, Schiemann Reaction), and azo compounds. Diazonium salts can react as pseudohalide-type electrophiles, and can therefore be used in specific protocols for the Heck Reaction or Suzuki Coupling.
The intermediates resulting from the diazotization of primary, aliphatic amines are unstable; they are rapidly converted into carbocations after loss of nitrogen, and yield products derived from substitution, elimination or rearrangement processes.
Mechanism of Diazotisation

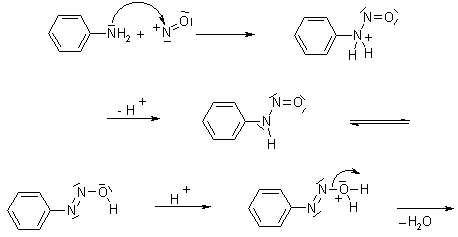

Dieckmann Condensation

The base-catalyzed intramolecular condensation of a diester. The Dieckmann Condensation works well to produce 5- or 6-membered cyclic ß-keto esters, and is usually effected with sodium alkoxide in alcoholic solvent.
The yields are good if the product has an enolizable proton; otherwise, the reverse reaction (cleavage with ring scission) can compete
The mechanism is similar to the Claisen Condensation
.
Diels-Alder Reaction


The [4+2]-cycloaddition of a conjugated diene and a dienophile (an alkene or alkyne), an electrocyclic reaction that involves the 4 π-electrons of the diene and 2 π-electrons of the dienophile. The driving force of the reaction is the formation of new σ-bonds, which are energetically more stable than the π-bonds.
In the case of an alkynyl dienophile, the initial adduct can still react as a dienophile if not too sterically hindered. In addition, either the diene or the dienophile can be substituted with cumulated double bonds, such as substituted allenes.
With its broad scope and simplicity of operation, the Diels-Alder is the most powerful synthetic method for unsaturated six-membered rings.
A variant is the hetero-Diels-Alder, in which either the diene or the dienophile contains a heteroatom, most often nitrogen or oxygen. This alternative constitutes a powerful synthesis of six-membered ring heterocycles.
Mechanism of the Diels-Alder Reaction


Overlap of the molecular orbitals (MOs) is required:
Overlap between the highest occupied MO of the diene (HOMO) and the lowest unoccupied MO of the dienophile (LUMO) is thermally allowed in the Diels Alder Reaction, provided the orbitals are of similar energy. The reaction is facilitated by electron-withdrawing groups on the dienophile, since this will lower the energy of the LUMO. Good dienophiles often bear one or two of the following substituents: CHO, COR, COOR, CN, C=C, Ph, or halogen. The diene component should be as electron-rich as possible.
There are "inverse demand" Diels Alder Reactions that involve the overlap of the HOMO of the dienophile with the unoccupied MO of the diene. This alternative scenario for the reaction is favored by electron-donating groups on the dienophile and an electron-poor diene.
The reaction is diastereoselective.




Cyclic dienes give stereoisomeric products. The endo product is usually favored by kinetic control due to secondary orbital interactions.

Huisgen Cycloaddition
1,3-Dipolar Cycloaddition
The Huisgen Cycloaddition is the reaction of a dipolarophile with a 1,3-dipolar compound that leads to 5-membered (hetero)cycles. Examples of dipolarophiles are alkenes and alkynes and molecules that possess related heteroatom functional groups (such as carbonyls and nitriles). 1,3-Dipolar compounds contain one or more heteroatoms and can be described as having at least one mesomeric structure that represents a charged dipole.
Examples of linear, propargyl-allenyl-type dipoles
An example of an allyl-type dipole.
Mechanism of the Huisgen 1,3-Dipolar Cycloaddition
2 π-electrons of the dipolarophile and 4 electrons of the dipolar compound participate in a concerted, pericyclic shift. The addition is stereoconservative (suprafacial), and the reaction is therefore a [2s+4s] cycloaddition similar to the Diels-Alder Reaction. Attention: many authors still use "[2+3] cycloaddition", which counts the number of involved atoms but does not follow IUPAC recommendations . IUPAC recommends the use of "(2+3)" for the number of involved atoms instead.
A condition for such a reaction to take place is a certain similarity of the interacting HOMO and LUMO orbitals, depending on the relative orbital energies of both the dipolarophile and the dipole. Electron-withdrawing groups on the dipolarophile normally favour an interaction of the LUMO of the dipolarophile with the HOMO of the dipole that leads to the formation of the new bonds, whereas electron donating groups on the dipolarophile normally favour the inverse of this interaction. Diazomethane as an electron-rich dipolar compound therefore rapidly reacts with electron-poor alkenes, such as acrylates. Relative reactivity patterns may be found in the literature (R. Huisgen, R. Grashey, J. Sauer in Chemistry of Alkenes, Interscience, New York, 1964, 806-877.).
Fischer Esterification
Fischer-Speier Esterification
The Lewis or Brønstedt acid-catalyzed esterification of carboxylic acids with alcohols to give esters is a typical reaction in which the products and reactants are in equilibrium.
The equilibrium may be influenced by either removing one product from the reaction mixture (for example, removal of the water by azeotropic distillation or absorption by molecular sieves) or by employing an excess of one reactant.


