Williamson Synthesis

This method is suitable for the preparation of a wide variety of unsymmetric ethers. The nucleophilic substitution of halides with alkoxides leads to the desired products.
If the halides are sterically demanding and there are accessible protons in the β-position, the alkoxide will act as a base, and side products derived from elimination are isolated instead.
Mechanism of the Williamson Synthesis


Wittig Reaction

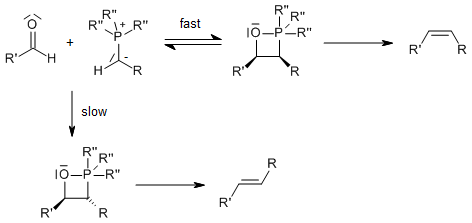
The Wittig Reaction allows the preparation of an alkene by the reaction of an aldehyde or ketone with the ylide generated from a phosphonium salt. The geometry of the resulting alkene depends on the reactivity of the ylide. If R is Ph, then the ylide is stabilized and is not as reactive as when R = alkyl. Stabilized ylides give (E)-alkenes whereas non-stabilized ylides lead to (Z)-alkenes
Mechanism of the Wittig Reaction
Addition of the ylide to the carbonyl is postulated to lead first to the zwitterionic intermediate betaine, which would then close to form a four-membered cyclic intermediate, an oxaphosphetane. The existence of the betaine hasn't been fully established, although its intermediacy plays an important role in the Schlosser Modification. Betaines may be stabilized by lithium salts leading to side products; therefore, suitable bases in the Wittig Reaction are for example: NaH, NaOMe, NEt3).

The driving force is the formation of a very stable phosphine oxide:

Reactive ylides give rapid reaction and subsequent rapid ring opening to give the (Z)-alkene:
Wolff-Kishner Reduction
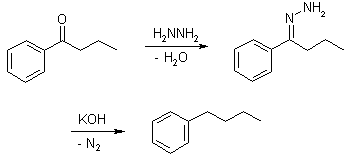
The reduction of aldehydes and ketones to alkanes. Condensation of the carbonyl compound with hydrazine forms the hydrazone, and treatment with base induces the reduction of the carbon coupled with oxidation of the hydrazine to gaseous nitrogen, to yield the corresponding alkane.
The Clemmensen Reduction can effect a similar conversion under strongly acidic conditions, and is useful if the starting material is base-labile.
Mechanism of the Wolff-Kishner Reduction

Wolff Rearrangement
The Wolff Rearrangement allows the generation of ketenes from α-diazoketones. Normally, these ketenes are not isolated, due to their high reactivity to form diketenes.
Wolff rearrangements that are conducted in the presence of nucleophiles generate derivatives of carboxylic acids, and in the presence of unsaturated compounds can undergo [2+2] cycloadditions
The formation of α-diazoketones from carboxylic acids (via the acyl chloride or an anhydride) and the subsequent Wolff Rearrangement in the presence of nucleophiles results in a one-carbon homologation of carboxylic acids. This reaction sequence, which first showed the synthetic potential of the Wolff-Rearrangement, was developed by Arndt and Eistert.
Mechanism of the Wolff Rearrangement
α-Diazoketones undergo the Wolff Rearrangement thermally in the range between room temperature and 750 °C in gas phase pyrolysis. Due to competing reactions at elevated temperatures, the photochemical and metal-catalyzed variants that feature a significantly lowered reaction temperature are often preferred (Zeller, Angew. Chem. Int. Ed., 1975, 14, 32. DOI).Nitrogen extrusion and the 1,2-shift can occur either in a concerted manner or stepwise via a carbene intermediate:
Silver ion catalysis fails with sterically hindered substrates, pointing to the requisite formation of a substrate complex with the ion. In these cases, photochemical excitation is the method of choice.
The solvent can affect the course of the reaction. If Wolff-Rearrangements are conducted in MeOH as solvent, the occurrence of side products derived from an O-H insertion point to the intermediacy of carbenes:
The course of the reaction and the migratory preferences can depend on the conditions (thermal, photochemical, metal ion catalysis) of the reaction. Analysis of the product distribution helps to determine different degrees of concertedness or the migratory aptitude of the group that rearranges. If R is phenyl, the main product comes from the rearrangement, whereas the methyl group gives more of the insertion side product.
The reactions of 2-diazo-1,3-diones also help to determine the migratory aptitude:
In a photolysis, methyl is preferred for rearrangement, whereas under thermolysis conditions the phenyl substituent migrates preferentially. Hydrogen always exceeds the migratory aptitude of phenyl groups. The alkoxy group in aryl or alkyl 2-diazoketocarboxylates never migrates.
Wurtz Reaction

The Wurtz Coupling is one of the oldest organic reactions, and produces the simple dimer derived from two equivalents of alkyl halide. The intramolecular version of the reaction has also found application in the preparation of strained ring compounds:
Using two different alkyl halides will lead to an approximately statistical mixture of products. A more selective unsymmetric modification is possible if starting materials have different rates of reactivity
Mechanism of the Wurtz Reaction

Side products:

Wurtz-Fittig Reaction

This reaction allows the alkylation of aryl halides. The more reactive alkyl halide forms an organosodium first, and this reacts as a nucleophile with an aryl halide as the electrophile. Excess alkyl halide and sodium may be used if the symmetric coupled alkanes formed as a side product may be separated readily.


