Favorskii Reaction

The rearrangement of cyclopropanones, often obtained as intermediates from the base-catalyzed reaction of α-halo ketones, leading to carboxylic acids and derivatives.
Mechanism of the Favorskii Reaction

Esters are obtained if alkoxide bases are used:

A direct conversion from α-halo ketones is possible:

Ring-contraction:
Fischer Indole Synthesis

The conversion of aryl hydrazones to indoles; requires elevated temperatures and the addition of Brønsted or Lewis acids. Some interesting enhancements have been published recently; for example a milder conversion when N-trifluoroacetyl enehydrazines are used as substrates
Mechanism of the Fischer Indole Synthesis

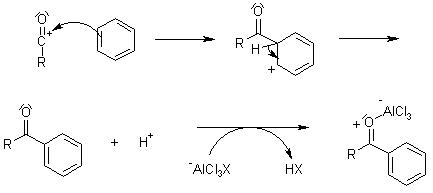
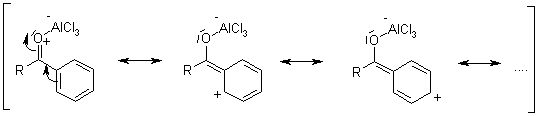
Friedel-Crafts Acylation

This electrophilic aromatic substitution allows the synthesis of monoacylated products from the reaction between arenes and acyl chlorides or anhydrides. The products are deactivated, and do not undergo a second substitution. Normally, a stoichiometric amount of the Lewis acid catalyst is required, because both the substrate and the product form complexes.
The Friedel-Crafts Alkylation may give polyalkylated products, so the Friedel-Crafts Acylation is a valuable alternative. The acylated products may easily be converted to the corresponding alkanes via Clemmensen Reduction or Wolff-Kishner Reduction.

Mechanism of the Friedel-Crafts Acylation


Friedel-Crafts Alkylation

This Lewis acid-catalyzed electrophilic aromatic substitution allows the synthesis of alkylated products via the reaction of arenes with alkyl halides or alkenes. Since alkyl substituents activate the arene substrate, polyalkylation may occur. A valuable, two-step alternative is Friedel-Crafts Acylation followed by a carbonyl reduction.
Mechanism of the Friedel-Crafts Alkylation


Using alkenes :

Fries Rearrangement

The Fries Rearrangement enables the preparation of acyl phenols.
Mechanism of the Fries Rearrangement
The reaction is catalyzed by Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4 or SnCl4. The acids are used in excess of the stoichiometric amount, especially the Lewis acids, since they form complexes with both the starting materials and products.

The complex can dissociate to form an acylium ion. Depending on the solvent, an ion pair can form, and the ionic species can react with each other within the solvent cage. However, reaction with a more distant molecule is also possible:

After hydrolysis, the product is liberated.

The reaction is ortho,para-selective so that, for example, the site of acylation can be regulated by the choice of temperature. Only sterically unhindered arenes are suitable substrates, since substituents will interfere with this reaction.
The requirement for equimolar quantities of the catalyst, the corrosive and toxic conditions (HF), and the violent reaction of the catalyst with water have prompted the development of newer protocols. Zeolites have proven to be unsuitable, since they are deactivated, but strong acids, such as sulfonic acids, provide a reasonable alternative.
An additional option for inducing a Fries Rearrangement is photochemical excitation, but this method is only feasible in the laboratory:

Gabriel Synthesis

Potassium phthalimide is a -NH2-synthon which allows the preparation of primary amines by reaction with alkyl halides. After alkylation, the phthalimid is not nucleophile and does not react anymore. Product is cleaved by reaction with base or hydrazine, which leads to a stable cyclic product.
Mechanism of the Gabriel Synthesis
Note: Phthalimide is acidic!


Cleavage:

Haloform Reaction

This reaction has been used in qualitative analysis to indicate the presence of a methyl ketone. The product iodoform is yellow and has a characteristic odour. The reaction has some synthetic utility in the oxidative demethylation of methyl ketones if the other substituent on the carbonyl groups bears no enolizable α-protons.
Mechanism of the Haloform Reaction
The reaction readily proceeds to completion because of the acidifying effect of the halogen substituents.

Hell-Volhard-Zelinsky Reaction

Treatment with bromine and a catalytic amount of phosphorus leads to the selective α-bromination of carboxylic acids.
Mechanism of the Hell-Volhard-Zelinsky Reaction
Phosphorus reacts with bromine to give phosphorus tribromide, and in the first step this converts the carboxylic acid into an acyl bromide.

An acyl bromide can readily exist in the enol form, and this tautomer is rapidly brominated at the α-carbon. The monobrominated compound is much less nucleophilic, so the reaction stops at this stage. This acyl intermediate compound can undergo bromide exchange with unreacted carboxylic acid via the anhydride, which allows the catalytic cycle to continue until the conversion is complete.

Hofmann Elimination

Sometimes referred to as the Hofmann Degradation. This elimination reaction of alkyl trimethyl amines proceeds with anti-stereochemistry, and is generally suitable for producing alkenes with one or two substituents. The reaction follows the Hofmann Rule.
Mechanism of the Hofmann Elimination


Knoevenagel Condensation
Doebner Modification

The condensation of carbon acid compounds with aldehydes to afford α,β-unsaturated compounds.
The Doebner Modification, which is possible in the presence of carboxylic acid groups, includes a pyridine-induced decarboxylation.
Mechanism of the Knoevenagel Condensation
An enol intermediate is formed initially:

This enol reacts with the aldehyde, and the resulting aldol undergoes subsequent base-induced elimination:

A reasonable variation of the mechanism, in which piperidine acts as organocatalyst, involves the corresponding iminium intermediate as the acceptor:
The Doebner-Modification in refluxing pyridine effects concerted decarboxylation and elimination:

Kolbe-Schmitt Reaction

A base-promoted carboxylation of phenols that allows the synthesis of salicylic acid derivatives.


